Introduction
The coronavirus disease 2019 (COVID-19) pandemic in late 2019 emerged in Wuhan, China, and has rapidly spread worldwide.1 India has reported a total of 2,81,75,044 confirmed COVID-19 cases and 3,31,895 deaths. Out of the total confirmed cases, over 7290 patients have been diagnosed with fungal infections, and over 219 deaths have occurred.2, 3 Notwithstanding the adoption of public health measures like public education, lockdown, social distancing, case & contact management to reduce disease transmission, it has massively spread in the human populaces all through the country, with new difficulties emerging in the clinical landscape.1, 4
In India, COVID-19 is already a matter of great concern, and suddenly Covid-triggered fungus infection (CTFI) pops up with a steady rise, thus raising a sense of alarm in the country.3, 5 The Covid-19 triggered fungal infection includes Mucormycosis (Zygomycosis) and Aspergillosis.1 The COVID-19 patient populace at the highest risk of CTFI is the elderly patients suffering from Adult Respiratory Distress Syndrome (ARDS), receiving broad-spectrum antibiotics, patients undergoing invasive or non-invasive ventilation, and those undergoing immunosuppressive or corticosteroid therapies.6, 7 There is a 3.33-fold increased risk of developing a CTFI in patients receiving corticosteroids than other patients who did not receive steroidal treatment.8
It is important to reinforce that there are other risk factors associated with severe cases of CTFI’s, such as malnutrition, prolonged intubation, central venous and arterial routes, nasogastric tube, persistent & continuous steam inhalation, diabetes, immunocompromised patients due to glucocorticoid therapy, immunotherapy, oncological diseases, haematological diseases, increased number of transplants, surgical procedures, individuals with AIDS, among others unclean and humid environments where the patients are treated and oxygenated, use of unsterile industrial grade oxygen, tap water in humidifiers & zinc supplement.5, 9, 10, 11
The reviewed literature on the postmortem findings in COVID-19 deaths has revealed substantial evidence about the pathogenesis of this disease. A systematic review study done on the postmortem pathologic findings has revealed diffuse alveolar damage as the most predominant finding in the lungs of COVID-19 patients who were assessed by autopsy. The study concluded that widespread pulmonary microthrombosis and extensive pulmonary angiogenesis, with frequent extrapulmonary microthrombotic and thromboembolic findings in patients with coronavirus disease, were consistent with the disease-specific disease hypercoagulability.12
Unfortunately, with the advent of the CTFI, a crucial invasive disease responsible for fatality, very few research papers have commented on the postmortem findings of invasive fungal infections affecting the rhino-orbital and craniocerebral structures in COVID-19 deaths. It is pertinent to say that an autopsy must establish the role of these CTFI’s concerning the cause of death in Covid-19 patients.13 This review research deals with autopsy dissection techniques and possible postmortem findings of invasive fungal infections involving the nasal and paranasal sinuses and orbital structures in COVID-19 deaths. The postmortem dissection of rhino-orbital structures is rarely done except in few indicated cases; many autopsy surgeons may not be suitably versed with the dissection techniques employed to look for such sites. Therefore, it is necessary to develop the autopsy protocols in investigating COVID-19 deaths associated with CTFI, such as considering the role of pre-and post-autopsy investigations, tissue samples required to preserve, and performing autopsies with minimal disfiguration.
Anatomy of Nasal Cavities
The nasal bones and rhino-orbital structures are rarely examined in routine autopsies, whether medicolegal or pathological. However, a forensic pathologist must be well aware of the anatomical aspects of these structures for easy access while performing the autopsy whenever indicated. The skeletal framework of nasal cavities is made by paired (nasal, maxillary, palatine & lacrimal) and unpaired (ethmoid, sphenoid, frontal & vomer) bones. The two nasal cavities are the commencing parts of the respiratory tract, with an extensive inferior base and a superior conical apex. The nasal cavity is separated from the oral by hard palate while the parts of the frontal, ethmoid and sphenoid bones separates it from the cranial cavity placed above. Lateral to it are the orbits (Figure 1). The nose in its lateral wall has three arched conchae bones placed above each other and projects medially and inferiorly across the nasal cavity. These conchae divide each nasal cavity into four chambers: three meatuses (superior, inferior and middle) and spheno-ethmoidal recess.
The Ethmoid Bone
The ethmoid is the critical element of all bones contributing to the skeletal structure of the nasal cavity (Figure 2). It forms the roof, lateral and medial wall of both the nasal cavities and orbits and contains the ethmoidal cells forming part of the anterior cranial fossa.14 Thus, the bone ethmoid comprises three parts – the perpendicular plate, the cribriform plate, and the ethmoidal labyrinth.
The cribriform plate is a perforated sheet of bone forming the roof of the nasal cavity penetrated by numerous olfactory nerve fibres. It fills the separates the nasal cavities below from the cranial cavity above. A triangular process (the crista Galli) at the midline on the superior surface of the cribriform plate anchors a fold (falx cerebri) of dura mater in the cranial cavity.
The perpendicular plate drops down vertically in the median sagittal plane from the cribriform plate to form part of the nasal septum.
The ethmoidal labyrinths, which sandwich between the ethmoidal cells, consist of an orbital plane forming the medial wall of the orbit. The medial sheet forms the upper part of the lateral wall of the nasal cavity.
Paranasal Sinuses
They are air-containing extensions of the nasal cavity and are lined by ciliated pseudostratified epithelium, interspersed with mucus-secreting goblet cells. The nasal cavity erodes into the surrounding bones during development, creating these sinuses. Therefore, all the sinuses drain back into the nasal cavity through their openings on the roof and lateral nasal walls.15
Frontal sinuses are triangular, variable in size, most superior, and drains into the lateral wall of the middle meatus via the frontonasal duct.
Sphenoidal sinuses are present in the body of sphenoid bone and open into the roof of the nasal cavity via apertures on the posterior wall of the spheno-ethmoidal recess.
Ethmoidal sinuses fill the ethmoidal labyrinth, three in number, and open into the ethmoidal infundibulum, lateral wall of the middle meatus, and lateral wall of the superior nasal meatus.
Maxillary sinuses are the most enormous filling the maxillary body. They are pyramidally located slightly inferior to the nasal cavities and drain into the nasal cavity at the top of the base, underneath the frontal sinus opening. This is a potential pathway for the spread of infection – fluid draining from the frontal sinus can enter into this sinus.
Etiopathogenesis of Mucormycosis
Mucormycosis is caused by fungi belonging to the order Mucorales, divided into six families.9, 16 The Mucorales are a common environmental fungus to which people are regularly exposed. Infection with these fungi is primarily seen in persons with diabetes, defective phagocytic function (e.g., neutropenia or glucocorticoid therapy) with high levels of free iron promote fungal growth in blood and tissues.17 Inhalation, ingestion, or the deposition of spores in wounds are all ways to become infected with Zygomycetes. As a result of their invasion and proliferation inside the lumen and walls of significant blood arteries, the fungi cause pathogenic lesions, culminating in ischemia and tissue necrosis. Zygomycotic fungi prefer the interior elastic lamina of arterial blood vessels, whereby they disseminate by angioinvasion.
As the fungus germinates, it might migrate cranially to the brain, inferiorly to the palate, laterally to the orbits and posteriorly to the sphenoid sinus and beyond into the cavernous sinus. The fungus has a propensity for growing all along elastic lamina of vessel walls, separating it from the media. The fungus’s direct infiltration and segmentation produce significant endothelial damage, leading to thrombus and ischemia in the surrounding tissues. The infarcted tissue produces an environment that encourages fungal multiplication, and the resulting lack of vascular supply makes systemic medical treatments ineffective in eliminating the fungus. The vessels infiltrate the nasal and sinus walls, and the orbit is invaded subsequently by freely connected foramen and venous channels. Finally, the fungus enters the skull by the orbital apex or the ethmoid's cribriform plate, eventually killing the patient.18, 19
Figure 1
Nasal Cavities (anterolateral view) Source:(Tibbits, R., & Richardson, P. (2005). Nasal Cavities (anterolateral view) [Illustration]. In Gray’s Anatomy for Students (2nd ed., p. 1013).14

Figure 2
Coronal Section through the Skull Source: (Tibbits, R., & Richardson, P. (2005). Coronal Section through Skull [Illustration]. In Gray’s Anatomy for Students (2nd ed., p. 1017).16
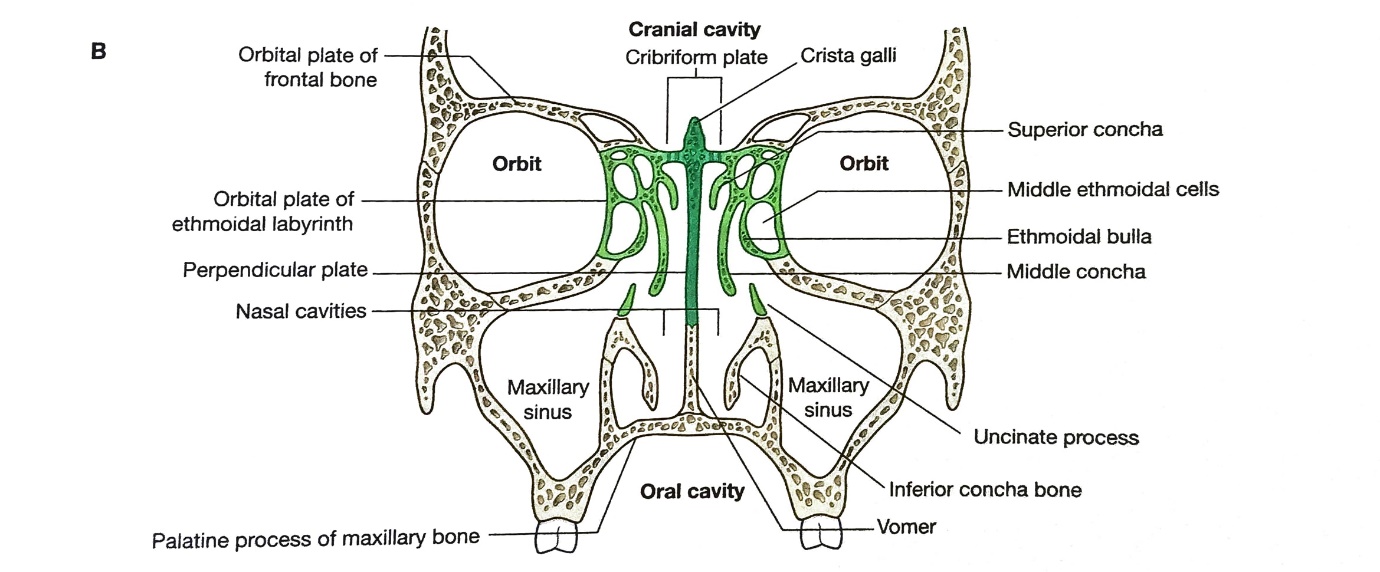
Figure 3
Showing A. Cribriform Plate for approaching ethmoidal sinus by chiselling. B. The lines indicate the saw cuts’ position when removing the right eye from an internal (superior) approach. (Picture: AIIMS, Patna, Bihar, India)
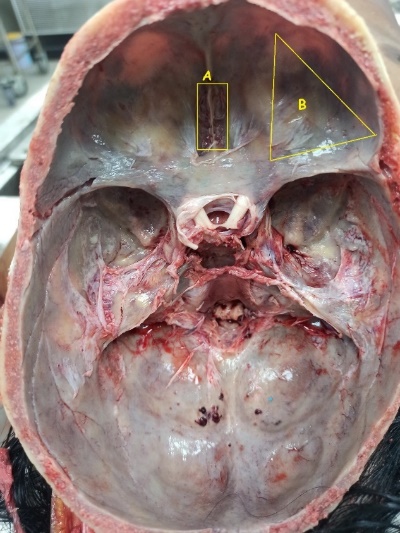
Figure 4
Microphotograph of mucor of the nasal wall showing numerous hyphae, which stains with haematoxylin (Michaelis, L., & B. Hellquist, 2003) H. (2003) (In-Ear, Nose, and Throat Histopathology (Second ed., p. 159)20

Figure 5
Microphotograph of Aspergillosis of the maxillary antrum. The Aspergillus is seen as a tangled mass of branching septate hyphae. Necrotic inflammatory cells, predominantly neutrophils, are seen above left. Periodic acid-Schiff stain (Michaelis L and B Hellquist, 2003). [Photograph]. In Ear, Nose and Throat Histopathology (Second ed., p. 157). (Michaelis, L., & B. Hellquist, H. (2003).21

Figure 6
Microphotograph showing branching fungal hyphae in inspissated mucus. Grocott methenamine silver stain (Michaelis, L., & B. Hellquist, H. (2003). In Ear, Nose and Throat Histopathology (Second ed., p. 155)

Figure 7
Microphotographof Mucorales and Aspergillus. Mucorales can be distinguished from Aspergillus by the presence of large, pauci septate, ribbon-like hyphae (A). In contrast, narrow,septate hyphae with acute angle branching are characteristic of Aspergillus (C). Mucorales may have less intense Grocott methenamine silver (GMS)positivity than Aspergillus (B and D). (Haematoxylin-eosin, magnification x400[A and C], and GMS, magnification x400 [B and D]). (Halvorson TS et al., 2020)(Halvorson, T. S., Isaacson, A. L., Ford, B. A., & Firchau, D. J. (2020).[Photograph]. In The Postmortem Features of Mucormycosis (pp. 72–80).

Figure 8
Photograph of necropsy specimen of the nose and paranasal sinuses showing Aspergillus in the right ethmoid and sphenoidal sinuses and orbit. Destruction of the cribriform plate is visible. C = Cribriform plate, E = ethmoid sinuses, F = frontal sinus, 0 = orbit, S = sphenoid sinus. (Milroy C et al., 1989) Aspergillosis of Nose and Paranasal Sinuses

Diagnosis of Invasive Fungal Infection11, 22
The intriguing fungal infection linked to COVID-19 disease can be diagnosed by investigations like nasal endoscopy, radio-imaging, histopathology of tissue samples and microbial culture. The diagnostic nasal endoscopy reveals discolouration of mucosa (either darkened or pale), showing crusts, debris, scabs, granulation, reduced bleeding, and insensate mucosa. Exploration of maxillary sinus reveals a ball of fungus containing semisolid cheesy-white or blackish material. Computed tomography (CT) scan of nose and paranasal sinuses shows black necrotic mass, erosion and thinning of bones, masticatory muscles enlargement, and mucosal thickening of sinuses. Pulmonary fungal infection can be diagnosed by lung, which reveals i. Halo sign in the form of a ring of ground-glass opacity surrounding nodular infiltrate, which pathophysiologically represents a region of ischemia, typical invasive pulmonary Aspergillosis, and ii. The reverse halo sign is also known as inverse halo or atoll sign, an area of ground-glass opacity surrounded by a consolidation ring. The MRI scan of the brain done depending on the structures associated shows optic neuritis, intracranial involvement, cavernous sinus thrombosis and infratemporal fossa involvement. The culture from mucosal swabs collected and inoculated in routine media at 30o C and 37o C will show cottony white or greyish black colony of mucormycosis.
Autopsy in CTFI Deaths
The autopsies in deaths due to invasive fungal infection related to COVID-19 diseases are mostly pathological, or research autopsies may be done with the permission or consent of the deceased’s next of kin. Rarely are they forensic or medicolegal unless an element of the unnatural cause or medical negligence is involved in the causation of death. Before proceeding with the autopsy per se, an invasive fungal infection diagnosis could be made by pre-autopsy information like the decedent’s medical history, radiological imaging, and laboratory investigations. However, in the absence of the above information or possible litigation involved, it may require examining nasal and paranasal structures at autopsy to identify these lesions. Therefore, the objectives of the autopsy in CTFI deaths primarily are to explore the sinuses for diagnosing the invasive fungal infection, to collect and preserve the tissue samples/scrapings for histopathology and microbiology investigations. Also, it is essential to establish the role of these CTFI’s in causing the death of COVID-19 patients that the autopsy examination can achieve.
Autopsy Technique for Dissection of Nose and Paranasal Sinuses
In conventional autopsy, rhino-orbital and paranasal sinuses are not involved in the dissection technique. However, in a pathological autopsy, the dissection may include exploring rhino-orbital structures for the gross observation of invasive fungal infection.
As the nose and paranasal sinuses are an extensive system of bones and air-containing spaces near the face, it is essential to preserve the external appearances of this area after autopsy to avoid disfiguration of the face.23 Therefore, a limited cosmetic approach of dissection by restricting the incision to the relevant face area may be the only option available. Thus, two techniques of postmortem dissection of nasal and paranasal sinuses were identified, i. the Coronal Saw-cut technique and ii. the Intracranial approach.
Coronal Saw-cut Technique11
After removing the skull cap and the brain, a coronal saw cut is made via the whole width of the skull base. This should be made in front of the vertebral bodies but behind the condyle of the mandible. This dissection will expose the superior wall of the nasopharynx, where paranasal sinuses and nose can be examined. The palatal part of the maxilla is usually left in situ if the infection is situated in the roof of the maxillary sinus. If the orbital floor is involved, an orbital exenteration is carried out in continuity with the specimen. If the infection extends to the maxillary sinus floor only, the orbital contents can be saved, but the palatal part of the maxilla is usually removed. This approach requires excellent surgical skills to carry the dissection without disturbing the nasopharyngeal structures of interest. Post dissection, after observing the lesions and taking swabs for culture or HPE, the two parts of the skull should fit together with fine suturing along with the lateral parts of the face and the neck.
The Intracranial Approach24
This approach is best suited for exploring paranasal sinuses through the skull base. The merits of this technique are it gives easy access to these structures, is less cumbersome and can be done quickly by any experienced autopsy surgeon. Also, the mutilation is limited and restricted to the inner cranial cavity. After removing the skull cap and the brain, the base of the skull is exposed. With the help of a chisel and a mallet, the cribriform plate in the anterior cranial fossa is chiselled till the maxillary sinus is reached. Alternatively, the orbital contents, including the eyeball, is removed by breaking the bone of the anterior cranial fossa. The maxillary antrum is accessed via the floor of the orbit. Next, the sphenoid sinus is exposed after exposing the anterior and posterior base of the pituitary fossa. Since the frontal sinus is located anteriorly, continued chiselling of the rear walls of the frontal sinus close to the midline will reveal the interior of this sinus.
Autopsy Dissection Techniques for Orbital Contents 24
There are two techniques for dissecting the orbital contents anterior approach and intracranial approach. First, an incision of the periorbita will reveal a layer of normal tissue on orbital exenteration followed by a deeper venous structure thrombosed with black-appearing discolouration.25 The stepwise dissection in these approaches is depicted in the flowchart below:
Autopsy Sample Collection and Preservation for the Diagnosis of CTFI26, 27, 28
The samples to be collected and preserved for histopathology and mycological studies are scrapings and exudate from the nose, lesions from the hard palate, sinus material, biopsy tissue from extracted tooth socket area, and debrided tissues. The method of collection and preservation is as below:
All precautions like using Personal Protective Equipment’s (PPE) kits, double-layered N 95 masks, etc., should be used while conducting the autopsy.
All specimens should be collected in sterile containers and transported to the laboratory within 2 hours. Avoid sending sample swabs if pus or sterile body fluids can be aspirated or when tissues are obtained. Dry swabs should not be used to collect these samples.
All the tissue that appears to be infected is collected and fixed using the paraffin wax layer.
Request for fungal culture should be made of the tissue samples collected and preserved in saline.
Request GMS (Grocott methenamine silver) stain for histopathological examination of the tissue samples collected. These should be collected in 10% formalin. It is based on the principle that mucopolysaccharide components of the fungal cell wall, when oxidized, release aldehyde groups. The aldehyde groups then react with the silver nitrate, reducing it to metallic silver, rendering them visible. In addition, it imparts a black colour to the fungal profiles and pale green colour to the background.
Gross and Histopathology Features of Invasive Fungal Infection
Mucormycosis mainly affects the sphenoidal sinuses. It appears a pale grey, hard tissue that involves the mucosa of the nose and paranasal sinuses and widely infiltrates the tissues of the face and orbit. The bones of the nose show extensive necrosis and abscesses (Figure 7). Histopathologically, it shows haemorrhagic infarction, coagulation necrosis, angioinvasion, infiltration by neutrophils (in non-neutropenic hosts), and perineural invasion. The hyphae, Rhizopus Oryza of the class Mucorales, are broad, show infrequent septae, and have non-parallel sides. They are often basophilic and stain deeply with haematoxylin (Figure 3). Sporangia are seen on rare occasions.11
Aspergillosis mainly involves the maxillary sinus. Grossly it appears like a soft mass of variable colour, which lies within the lumen or is attached to the sidewall of the antrum. Histopathologically, it shows tangled mycelium of aspergilli with a thin coating of inflammatory cells containing neutrophils, lymphocytes, and multinucleate giant cells (Figure 4, Figure 6). A granulomatous inflammatory reaction represents the central part of the lesion and is composed of giant cells, histiocytes, neutrophils, and eosinophils with much fibrosis. The aspergilli are mainly to be found in the cytoplasm of the giant cells. Staining with GMS and PAS (Periodic Acid-Schiff) shows the hyphae, which characteristically are septate and branch at an acute angle of about 45° (Figure 5, Figure 6). The fungal balls of the paranasal sinuses frequently contain short, bizarrely shaped hyphae. Conidiophores are not seen in the tissues.11
Conclusion
Covid-19 triggered fungal infections (CTFIs) are rapidly turning into an epidemic disease, affecting a large population of COVID patients. Many deaths are attributed to this invasive fungal infection, affecting mainly vital structures such as the brain and orbits. Performing autopsy in fatalities due to COVID-19 disease and the findings observed has played a crucial role in understanding the pathogenesis of the disease. Although the COVID-19 infection poses an imminent threat to autopsy surgeons and mortuary staff, restriction of autopsies in COVID-19 deaths in general with further limitations on pathological autopsies impedes the advancement of knowledge one can gain by conducting them. Minimally invasive and less mutilating techniques procedures may offer an excellent aid for conducting autopsies in such highly infectious diseases. Though these postmortem techniques cannot replace the timely honoured complete medicolegal autopsy, they can be helpful in restrictive pathological autopsies and highly contagious diseases like COVID-19. In the era of a highly infectious pandemic where postmortem examination is not advised, autopsy surgeons need to adopt newer advanced techniques to identify the disease etiopathogenesis and fulfil the objectives of postmortem examinations.

